Carboxylic acids are organic acids that have the functional group. Despite having the same functional groups as both a carbonyl and an alcohol, the carboxylic acid shows few similarities to that of aldehydes and ketones or primary alcohols.
Carboxylic acids have the suffix -anoic after there name.For example an acid with 3 carbons in it's chain would be called propanoic acid. Traditional name are often used for the common acids ;
e.g Methanoic acid is commonly known as formic acid
Ethanoic acid is known as acetic acid (major ingrediant of vinegar)
Physical properties
Carboxylic acids are able to hydrogen bond between molecules and also to water, making them very soluble. Although the boiling point of the acids is high compared to hydrocarbons of similar RMM it is not as high as expected as the acids are able to form dimers; that is they form in pairs resembling a six pointed ring. Carboxylic acids have a very unpleasant smell which is in contrast to the sweet, fruity smell of the esters which they are isometric with.

This is picture of Methanoic acid, the smallest carboxyllic acid.
Chemical properties
Carboxylic acids are formed by the oxidation of primary alcohols or aldehydes. They will not oxidize further chemically, but will burn in air to form CO2 and H2O.Most acids are weak compared to mineral acids such hydrochloric acid.The strength of an organic acid depends on the constituents near the crboxyl group. For example if an electron withdrawing group such as a chloride group was attatched to a carbon near the carboxyl group then this would 'pull' electron charge away from the O-H group and hence reduce the hold on the H of the O-H group (it is the ability to lose this proton that makes for the acidity of a solution of the compound). Carboxylic acids can be reduced to primary alcohols by a strong reducing agent such as LiAlH4 in dry ethoxyethane.
Some of the more important reactions of Carboxylic acids:

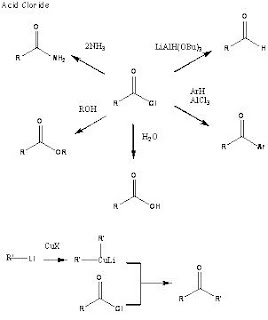
One of the most important reactions of carboxylic acids is the conversion to an acid halide.
Acid Halides
Acid chlorides are prepared from the corresponding carboxylic acids by thionyl chloride, phosphorus trichloride or phosphorus pentachloride. They have the same structure as carboxylic acids but the O-H of the acid is replaced by a halide.

Acid chlorides are the most reactive of the carboxylic acid derivatives. Acid chlorides are very unstable in moist conditions and give off HCl gas on reaction with water.
Some of the important reactions of acid chlorides:
Acid anhydrides
Organic anhydrides are colourless liquids regarded as being formed from from two molecules of carboxylic acid by the removal of a water molecule.
They are usually prepared by distilling a mixture of the acid chloride and the sodium salt of the carboxylic acid in a dry apparatus and collecting the anhydride as the distillate.
Acid anhydrides are less reactive than acid chlorides and are used to prepare amides and esters, especially of phenols where direct esterification is not possible. Acid anhydrides are hydrolyzed to there parent carboxylic acids with water (as shown below).